The Sars Virus Is a Member of the Family of Viruses
The nowadays outbreak of a coronavirus-associated acute respiratory illness called coronavirus disease 19 (COVID-19) is the third documented spillover of an animal coronavirus to humans in only two decades that has resulted in a major epidemic. The Coronaviridae Study Group (CSG) of the International Committee on Taxonomy of Viruses, which is responsible for developing the classification of viruses and taxon nomenclature of the family unit Coronaviridae, has assessed the placement of the homo pathogen, tentatively named 2019-nCoV, within the Coronaviridae. Based on phylogeny, taxonomy and established practice, the CSG recognizes this virus as forming a sis clade to the prototype human and bat astringent acute respiratory syndrome coronaviruses (SARS-CoVs) of the species Astringent acute respiratory syndrome-related coronavirus, and designates it as SARS-CoV-2. In club to facilitate communication, the CSG proposes to apply the post-obit naming convention for private isolates: SARS-CoV-2/host/location/isolate/date. While the full spectrum of clinical manifestations associated with SARS-CoV-2 infections in humans remains to exist determined, the independent zoonotic transmission of SARS-CoV and SARS-CoV-2 highlights the demand for studying viruses at the species level to complement research focused on individual pathogenic viruses of immediate significance. This will improve our understanding of virus–host interactions in an ever-changing environment and raise our preparedness for futurity outbreaks.
Upon a viral outbreak, it is important to quickly plant whether the outbreak is caused by a new or a previously known virus (Box 1), as this helps decide which approaches and actions are most appropriate to detect the causative agent, control its transmission and limit potential consequences of the epidemic. The assessment of virus novelty also has implications for virus naming and, on a different timescale, helps to define enquiry priorities in virology and public health.
For many human virus infections such equally influenza virus1 or norovirus2 infections, well-established and internationally approved methods, standards and procedures are in place to identify and name the causative agents of these infections and report this data promptly to public wellness authorities and the full general public. In outbreaks involving newly emerged viruses, the situation may be dissimilar, and appropriate procedures to deal with these viruses need to be established or refined with loftier priority.
Here, we nowadays an assessment of the genetic relatedness of the newly identified human coronavirus3, provisionally named 2019-nCoV, to known coronaviruses, and detail the ground for (re)naming this virus severe acute respiratory syndrome coronavirus two (SARS-CoV-2), which will be used future. Given the public interest in naming newly emerging viruses and the diseases caused by these viruses in humans, we will give a brief introduction to virus discovery and classification — specifically the virus species concept — and the roles of dissimilar bodies, such as the World Health Organization (WHO) and the International Committee on Taxonomy of Viruses (ICTV), in this process. We promise this will help readers to better empathise the scientific approach we have taken to arrive at this proper noun, and we will likewise hash out implications of this analysis and naming determination.
Classifying and naming viruses and virus species
Defining the novelty of viruses is one of the topics that virus nomenclature deals with. The nomenclature of RNA viruses needs to consider their inherent genetic variability, which ofttimes results in two or more viruses with non-identical only similar genome sequences beingness regarded as variants of the same virus. This immediately poses the question of how much difference to an existing group is large plenty to recognize the candidate virus as a member of a new, distinct group. This question is answered in best practice by evaluating the degree of relatedness of the candidate virus to previously identified viruses infecting the aforementioned host or established monophyletic groups of viruses, often known as genotypes or clades, which may or may non include viruses of unlike hosts. This is formally addressed in the framework of the official nomenclature of virus taxonomy and is overseen and coordinated by the ICTV4. Viruses are clustered in taxa in a hierarchical scheme of ranks in which the species represents the everyman and nearly populous rank containing the to the lowest degree diverged groups (taxa) of viruses (Box 2). The ICTV maintains a Study Grouping for each virus family. The Study Groups are responsible for assigning viruses to virus species and taxa of higher ranks, such every bit subgenera, genera and subfamilies. In this context they play an important role in advancing the virus species concept and highlighting its significance5.
Virus nomenclature is a formal system of names used to label viruses and taxa. The fact that there are names for almost all viruses within a species is due to the historical perception of viruses as causative agents of specific diseases in specific hosts, and to the way we commonly catalogue and classify newly discovered viruses, which increasingly includes viruses that have not been linked to any known illness in their respective hosts (Box 1). The WHO, an agency of the United Nations, coordinates international public health activities aimed at combating, containing and mitigating the consequences of infectious disease—including major virus epidemics—and is responsible for naming illness(s) caused by newly emerging human viruses. In doing and then, the WHO oft takes the traditional approach of linking names of specific diseases to viruses (Box ane) and assessing virus novelty by an apparent failure to detect the causative agent using established diagnostic assays.
Apart from disease, geography and the organism from which a given virus was isolated also dominate the nomenclature, occasionally engraving connections that may be accidental (rather than typical) or even stigmatizing, which should exist avoided. Establishing a universal nomenclature for viruses was ane of the major tasks of the ICTV when it was founded more than 50 years agone4. When the species rank was established in the taxonomy of viruses6, ICTV's responsibility for naming viruses was shifted to naming and establishing species. ICTV Written report Groups may likewise be involved in virus naming on a case-by-case basis as an extension of their official remit, as well as using the special expertise of their members. As virus species names are oft very similar to the name of the founding fellow member of the corresponding species, they are oft confused in the literature with names of private viruses in this species. The species name is italicized, starts with a capital alphabetic character and should not be spelled in an abbreviated grade7; hence the species proper noun Severe astute respiratory syndrome-related coronavirus. In contrast, this convention does not use to virus names, hence astringent astute respiratory syndrome coronavirus, or SARS-CoV, as it is widely known.
Defining the place of SARS-CoV-2 within the Coronaviridae
Researchers studying coronaviruses—a family of enveloped positive-strand RNA viruses infecting vertebrateseight—accept been confronted several times with the need to define whether a newly emerged virus causing a severe or even life-threatening disease in humans belongs to an existing or a new (however-to-exist-established) species. This happened with SARS9,ten,11,12 and with Middle East respiratory syndrome (MERS)13,fourteen a few years afterwards. Each fourth dimension, the virus was placed in the taxonomy using data derived from a sequence-based family classification15,16.
The current nomenclature of coronaviruses recognizes 39 species in 27 subgenera, five genera and ii subfamilies that belong to the family unit Coronaviridae, suborder Cornidovirineae, order Nidovirales and realm Riboviria 17,18,xix (Fig. i). The family classification and taxonomy are developed by the Coronaviridae Written report Group (CSG), a working grouping of the ICTV20. The CSG is responsible for assessing the identify of new viruses through their relation to known viruses in established taxa, including placements relating to the species Severe acute respiratorysyndrome-related coronavirus. In the nomenclature of nidoviruses, species are considered biological entities demarcated by a genetics-based method21, while by and large virus species are perceived as homo-made constructs22. To appreciate the difference between a nidoviral species and the viruses grouped therein, it may be instructive to expect at their relationship in the context of the full taxonomy structure of several coronaviruses. Although these viruses were isolated at different times and locations from different human and animal hosts (with and without causing clinical disease), they all belong to the species Severe acute respiratorysyndrome-related coronavirus, and their relationship parallels that between human individuals and the species Homo sapiens (Fig. 1).

Shown is the full taxonomy of selected coronaviruses in comparison with the taxonomy of humans (the founders of virology and other eminent scientists represent individual man beings for the sake of this comparing), which is given just for categories (ranks) that are shared with the virus taxonomy. Note that these ii taxonomies were independently adult using completely different criteria. Although no equivalence is implied, the species of coronaviruses is interpreted sensu stricto equally accepted for the species of humans.
Even without knowing anything about the species concept, every man recognizes another human as a fellow member of the same species. However, for assigning individual living organisms to nearly other species, specialized knowledge and tools for assessing inter-individual differences are required. The CSG uses a computational framework of comparative genomics23, which is shared by several ICTV Study Groups responsible for the classification and nomenclature of the order Nidovirales and coordinated by the ICTV Nidovirales Study Grouping (NSG)24 (Box three). The Study Groups quantify and sectionalisation the variation in the well-nigh conserved replicative proteins encoded in open up reading frames 1a and 1b (ORF1a/1b) of the coronavirus genome (Fig. 2a) to identify thresholds on pair-wise patristic distances (PPDs) that demarcate virus clusters at different ranks.
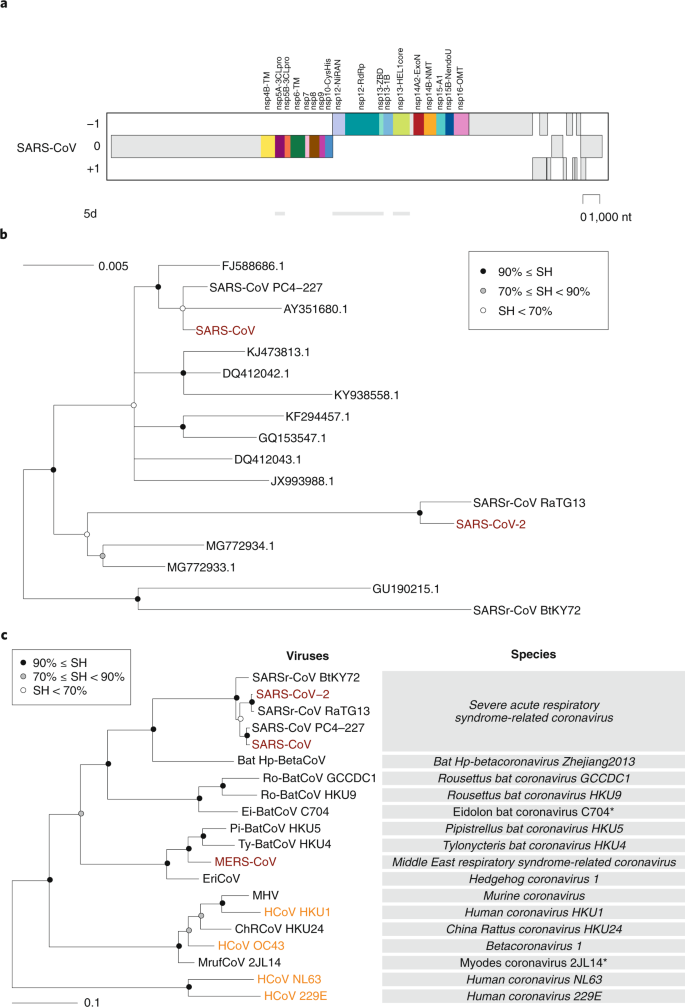
a, Concatenated multiple sequence alignments (MSAs) of the protein domain combination44 used for phylogenetic and DEmARC analyses of the family unit Coronaviridae. Shown are the locations of the replicative domains conserved in the ordert Nidovirales in relation to several other ORF1a/b-encoded domains and other major ORFs in the SARS-CoV genome. 5d, 5 domains: nsp5A-3CLpro, two beta-butt domains of the 3C-similar protease; nsp12-NiRAN, nidovirus RdRp-associated nucleotidyltransferase; nsp12-RdRp, RNA-dependent RNA polymerase; nsp13-HEL1 core, superfamily 1 helicase with upstream Zn-binding domain (nsp13-ZBD); nt, nucleotide. b, The maximum-likelihood tree of SARS-CoV was reconstructed by IQ‑TREE 5.one.6.one (ref. 45) using 83 sequences with the best fitting evolutionary model. Afterward, the tree was purged from the most like sequences and midpoint-rooted. Branch support was estimated using the Shimodaira–Hasegawa (SH)-similar approximate likelihood ratio test with 1,000 replicates. GenBank IDs for all viruses except 4 are shown; SARS-CoV, AY274119.three; SARS-CoV-2, MN908947.3; SARSr-CoV_BtKY72, KY352407.1; SARS-CoV_PC4-227, AY613950.one. c, Shown is an IQ‑TREE maximum-likelihood tree of single virus representatives of 13 species and five representatives of the species Severe acute respiratory syndrome-related coronavirus of the genus Betacoronavirus. The tree is rooted with HCoV-NL63 and HCoV-229E, representing two species of the genus Alphacoronavirus. Purple text highlights zoonotic viruses with varying pathogenicity in humans; orange text highlights common respiratory viruses that circulate in humans. Asterisks indicate 2 coronavirus species whose demarcations and names are pending approval from the ICTV and, thus, these names are not italicized.
Consistent with previous reports, SARS-CoV-2 clusters with SARS-CoVs in copse of the species Severe acute respiratory syndrome-related coronavirus (Fig. 2b) and the genus Betacoronavirus (Fig. 2c)25,26,27. Distance estimates between SARS-CoV-2 and the most closely related coronaviruses vary among different studies depending on the choice of measure (nucleotide or amino acid) and genome region. Appropriately, there is no agreement even so on the exact taxonomic position of SARS-CoV-two inside the subgenus Sarbecovirus. When we included SARS-CoV-2 in the dataset used for the most recent update (May 2019) of the coronavirus taxonomy currently beingness considered by ICTVxix, which includes 2,505 coronaviruses, the species composition was not afflicted and the virus was assigned to the species Severe acute respiratory syndrome-related coronavirus, as detailed in Box 4.
With respect to novelty, SARS-CoV-two differs from the ii other zoonotic coronaviruses, SARS-CoV and MERS-CoV, introduced to humans earlier in the twenty-commencement century. Previously, the CSG established that each of these two viruses paradigm a new species in a new informal subgroup of the genus Betacoronavirus fifteen,16. These two breezy subgroups were recently recognized as subgenera Sarbecovirus and Merbecovirus eighteen,28,29 when the subgenus rank was established in the virus taxonomyxxx. Being the first identified representatives of a new species, unique names were introduced for the two viruses and their taxa in line with the mutual practice and land of virus taxonomy at the corresponding times of isolation. The situation with SARS-CoV-2 is fundamentally unlike because this virus is assigned to an existing species that contains hundreds of known viruses predominantly isolated from humans and diverse bats. All these viruses accept names derived from SARS-CoV, although just the human isolates nerveless during the 2002–2003 outbreak have been confirmed to cause SARS in infected individuals. Thus, the reference to SARS in all these virus names (combined with the use of specific prefixes, suffixes and/or genome sequence IDs in public databases) acknowledges the phylogenetic (rather than clinical affliction-based) grouping of the respective virus with the prototypic virus in that species (SARS-CoV). The CSG chose the name SARS-CoV-two based on the established practice for naming viruses in this species and the relatively distant relationship of this virus to the prototype SARS-CoV in a species tree and the distance space (Fig. 2b and the effigy in Box 4).
The available yet limited epidemiological and clinical information for SARS-CoV-2 suggest that the disease spectrum and transmission efficiency of this virus31,32,33,34,35 differ from those reported for SARS-CoV9. To accommodate the wide spectrum of clinical presentations and outcomes of infections caused past SARS-CoV-ii (ranging from asymptomatic to severe or even fatal in some cases)31, the WHO recently introduced a rather unspecific proper noun (coronavirus disease 19, also known as COVID-19 (ref. 36)) to denote this disease. Also, the diagnostic methods used to confirm SARS-CoV-2 infections are not identical to those of SARS-CoV. This is reflected past the specific recommendations for public health practitioners, healthcare workers and laboratory diagnostic staff for SARS-CoV-two (for example, the WHO guidelines for SARS-CoV-2 (ref. 37). By uncoupling the naming conventions used for coronaviruses and the diseases that some of them cause in humans and animals, nosotros wish to support the WHO in its efforts to plant disease names in the near appropriate fashion (for further information, see the WHO's guidelines for affliction naming38). The further advancement of naming conventions is besides important considering the ongoing discovery of new human and animal viruses by side by side-generation sequencing technologies can be expected to produce an increasing number of viruses that exercise not (easily) fit the virus–illness model that was widely used in the pre-genomic era (Box 1). Having now established dissimilar names for the causative virus (SARS-CoV-2) and the disease (COVID-nineteen), the CSG hopes that this will raise awareness in both the full general public and public health authorities regarding the departure between these two entities. The CSG promotes this clear stardom because it will help improve the outbreak direction and also reduces the chance of confusing virus and disease, as has been the case over many years with SARS-CoV (the virus) and SARS (the disease).
To facilitate expert practise and scientific exchange, the CSG recommends that researchers describing new viruses (that is, isolates) in this species adopt a standardized format for public databases and publications that closely resembles the formats used for isolates of avian coronaviruses39, filoviruses40 and flu virus1. The proposed naming convention includes a reference to the host organism that the virus was isolated from, the place of isolation (geographic location), an isolate or strain number, and the time of isolation (twelvemonth or more detailed) in the format virus/host/location/isolate/date; for example, SARS-CoV-2/human/Wuhan/X1/2019. This complete designation along with additional and important characteristics, such as pathogenic potential in humans or other hosts, should be included in the submission of each isolate genome sequence to public databases such as GenBank. In publications, this name could exist further extended with a sequence database ID—for case, SARS-CoV-2/human/Wuhan/X1/2019_XYZ12345 (fictional case)—when first mentioned in the text. Nosotros believe that this format will provide critical metadata on the major characteristics of each detail virus isolate (genome sequence) required for subsequent epidemiological and other studies, besides every bit for control measures.
Expanding the focus from pathogens to virus species
Historically, public wellness and fundamental research have been focused on the detection, containment, handling and assay of viruses that are pathogenic to humans following their discovery (a reactive approach). Exploring and defining their biological characteristics in the context of the unabridged natural multifariousness as a species has never been a priority. The emergence of SARS-CoV-2 equally a human pathogen in December 2019 may thus exist perceived as completely contained from the SARS-CoV outbreak in 2002–2003. Although SARS-CoV-2 is indeed not a descendent of SARS-CoV (Fig. 2b), and the introduction of each of these viruses into humans was likely facilitated by independent unknown external factors, the two viruses are genetically so close to each other (Fig. 2c, panel c of the figure in Box 4) that their evolutionary histories and characteristics are mutually informative.
The currently known viruses of the species Astringent acute respiratory syndrome-related coronavirus may exist every bit (poorly) representative for this particular species as the few individuals that we selected to represent H. sapiens in Fig. 1. It is thus reasonable to assume that this biased noesis of the natural diversity of the species Severe astute respiratory syndrome-related coronavirus limits our electric current agreement of fundamental aspects of the biological science of this species and, as a consequence, our abilities to command zoonotic spillovers to humans. Future studies aimed at understanding the ecology of these viruses and advancing the accuracy and resolution of evolutionary analyses41 would benefit greatly from adjusting our enquiry and sampling strategies. This needs to include an expansion of our electric current inquiry focus on man pathogens and their adaptation to specific hosts to other viruses in this species. To illustrate the great potential of species-wide studies, it may again be instructive to draw a parallel to H. sapiens, and specifically to the impressive advancements in personalized medicine in contempo years. Results of extensive genetic analyses of large numbers of individuals representing diverse populations from all continents have been translated into clinical applications and greatly contribute to optimizing patient-specific diagnostics and therapy. They were instrumental in identifying reliable predictive markers for specific diseases as well as genomic sites that are under selection. Information technology thus seems reasonable to expect that genome-based analyses with a comparable species coverage will exist similarly insightful for coronaviruses. As well, boosted diagnostic tools that target the unabridged species should be developed to complement existing tools optimized to observe individual pathogenic variants (a proactive approach). Technical solutions to this trouble are already available; for example, in the context of multiplex PCR-based assays42. The costs for developing and applying (combined or separate) species- and virus-specific diagnostic tests in specific clinical and/or epidemiological settings may help to better capeesh the biological diversity and zoonotic potential of specific virus species and their members. Besides, the further reduction of time required to identify the causative agents of novel virus infections will contribute to limiting the enormous social and economical consequences of large outbreaks. To advance such studies, innovative fundraising approaches may be required.
Although this Consensus Statement focuses on a single virus species, the issues raised apply to other species in the family and possibly across. A commencement step towards appreciation of this species and others would be for researchers, journals, databases and other relevant bodies to adopt proper referencing to the full taxonomy of coronaviruses under study, including explicit mentioning of the relevant virus species and the specific virus(es) within the species using the ICTV naming rules explained above. This naming convention is, regretfully, rarely observed in common practice, with mixing of virus and species names being frequently found in the literature (including by the authors of this Consensus Argument on several past occasions). The adoption of authentic virus-naming practices should be facilitated by the major revision of the virus species nomenclature that is currently beingness discussed by the ICTV and is being planned for implementation in the well-nigh future43. With this change in identify, the CSG is resolved to address the existing pregnant overlap betwixt virus and species names that complicates the appreciation and use of the species concept in its awarding to coronaviruses.
References
-
Krammer, F. et al. Influenza. Nat. Rev. Dis. Primers 4, 3 (2018).
-
Zheng, D. P. et al. Norovirus classification and proposed strain nomenclature. Virology 346, 312–323 (2006).
-
Wu, A. et al. Genome composition and departure of the novel coronavirus (2019-nCoV) originating in Red china. Cell Host Microbe https://doi.org/ten.1016/j.chom.2020.02.001 (2020).
-
Adams, Chiliad. J. et al. fifty years of the International Committee on Taxonomy of Viruses: progress and prospects. Curvation. Virol. 162, 1441–1446 (2017).
-
Gorbalenya, A. Eastward., Lauber, C. & Siddell, S. Taxonomy of Viruses, in Reference Module in Biomedical Sciences (Elsevier, 2019) https://doi.org/10.1016/B978-0-12-801238-three.99237-7.
-
Van Regenmortel, M. H., Maniloff, J. & Calisher, C. The concept of virus species. Curvation. Virol. 120, 313–314 (1991).
-
ICTV Lawmaking. The International Code of Virus Classification and Nomenclature https://talk.ictvonline.org/information/due west/ictv-information/383/ictv-code (2018).
-
Masters, P. S. The molecular biology of coronaviruses. Adv. Virus Res. 66, 193–292 (2006).
-
Perlman, South. & Netland, J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 7, 439–450 (2009).
-
Drosten, C. et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967–1976 (2003).
-
Ksiazek, T. G. et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1953–1966 (2003).
-
Peiris, J. S. M. et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361, 1319–1325 (2003).
-
Zumla, A., Hui, D. S. & Perlman, S. Middle East respiratory syndrome. Lancet 386, 995–1007 (2015).
-
Zaki, A. One thousand., van Boheemen, S., Bestebroer, T. G., Osterhaus, A. D. M. E. & Fouchier, R. A. M. Isolation of a novel coronavirus from a human being with pneumonia in Kingdom of saudi arabia. Due north. Engl. J. Med. 367, 1814–1820 (2012).
-
Snijder, E. J. et al. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split up-off from the coronavirus group 2 lineage. J. Mol. Biol. 331, 991–1004 (2003).
-
van Boheemen, S. et al. Genomic label of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. mBio 3, e00473-12 (2012).
-
Siddell, S. One thousand. et al. Additional changes to taxonomy ratified in a special vote past the International Commission on Taxonomy of Viruses (October 2018). Arch. Virol. 164, 943–946 (2019).
-
Ziebuhr, J. et al. Proposal 2017.013S. A.v1. Reorganization of the family Coronaviridae into 2 families, Coronaviridae (including the current subfamily Coronavirinae and the new subfamily Letovirinae) and the new family Tobaniviridae (all-around the current subfamily Torovirinae and three other subfamilies), revision of the genus rank structure and introduction of a new subgenus rank. (ICTV, 2017); https://ictv.global/proposal/2017.Nidovirales/.
-
Ziebuhr, J. et al. Proposal 2019.021S.Ac.v1. Create x new species and a new genus in the subfamily Orthocoronavirinae of the family Coronaviridae and five new species and a new genus in the subfamily Serpentovirinae of the family Tobaniviridae. (ICTV, 2019); https://ictv.global/proposal/2019.Nidovirales/.
-
de Groot, R. J. et al. in Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses (eds Male monarch, A. M. Q. et al.) 806–828 (Elsevier Academic Press, 2012).
-
Lauber, C. & Gorbalenya, A. E. Toward genetics-based virus taxonomy: comparative analysis of a genetics-based classification and the taxonomy of picornaviruses. J. Virol. 86, 3905–3915 (2012).
-
Van Regenmortel, M. H. V. The species problem in virology. Adv. Virus Res. 100, one–eighteen (2018).
-
Lauber, C. & Gorbalenya, A. E. Partitioning the genetic variety of a virus family: arroyo and evaluation through a case study of picornaviruses. J. Virol. 86, 3890–3904 (2012).
-
Lauber, C. et al. Mesoniviridae: a new family in the order Nidovirales formed past a single species of mosquito-borne viruses. Arch. Virol. 157, 1623–1628 (2012).
-
Lu, R. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395, 565–574 (2020).
-
Zhou, P. et al. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Nature https://doi.org/x.1038/s41586-020-2012-7 (2020).
-
Zhu, Due north. et al. A novel coronavirus from patients with pneumonia in China, 2019. Due north. Engl. J. Med. 382, 727–733 (2020).
-
de Groot, R. J. et al. Centre Eastward respiratory syndrome coronavirus (MERS-CoV): declaration of the Coronavirus Study Grouping. J. Virol. 87, 7790–7792 (2013).
-
Gorbalenya, A. E., Snijder, E. J. & Spaan, W. J. Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 78, 7863–7866 (2004).
-
Gorbalenya, A. Eastward. et al. The new telescopic of virus taxonomy: partitioning the virosphere into xv hierarchical ranks. Nat. Microbiol. (in the press).
-
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, Mainland china. Lancet 395, 497–506 (2020).
-
Kui, 50. et al. Clinical characteristics of novel coronavirus cases in third hospitals in Hubei Province. Chin. Med. J. https://doi.org/10.1097/CM9.0000000000000744 (2020).
-
Li, Q. et al. Early on transmission dynamics in Wuhan, Cathay, of novel coronavirus-infected pneumonia. N. Engl. J. Med. https://doi.org/10.1056/nejmoa2001316 (2020).
-
Liu, Y., Gayle, A. A., Wilder-Smith, A. & Rocklov, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. https://doi.org/ten.1093/jtm/taaa021 (2020).
-
Tang, B. et al. Estimation of the manual run a risk of the 2019-nCoV and its implication for public wellness interventions. J. Clin. Med. 9, 462 (2020).
-
Novel Coronavirus (2019-nCoV) Situation Report – 22 (Earth Wellness System, 2020); https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf
-
Coronavirus disease (COVID-xix) outbreak (World Health Organization, 2020); https://world wide web.who.int/emergencies/diseases/novel-coronavirus-2019
-
Earth Health Organization best practices for the naming of new human being infectious diseases (World Health Organization, 2015); https://apps.who.int/iris/handle/10665/163636
-
Cavanagh, D. A nomenclature for avian coronavirus isolates and the question of species status. Avian Pathol. 30, 109–115 (2001).
-
Kuhn, J. H. et al. Virus nomenclature below the species level: a standardized classification for natural variants of viruses assigned to the family Filoviridae. Arch. Virol. 158, 301–311 (2013).
-
Forni, D., Cagliani, R., Clerici, K. & Sironi, M. Molecular evolution of homo coronavirus genomes. Trends Microbiol. 25, 35–48 (2017).
-
Nijhuis, R. H. T. et al. PCR assays for detection of human astroviruses: In silico evaluation and blueprint, and in vitro application to samples nerveless from patients in the netherlands. J. Clin. Virol. 108, 83–89 (2018).
-
Siddell, South. G. et al. Binomial nomenclature for virus species: a consultation. Arch. Virol. 165, 519–525 (2020).
-
Gorbalenya, A. E. et al. Practical application of bioinformatics by the multidisciplinary VIZIER consortium. Antiviral Res. 87, 95–110 (2010).
-
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and constructive stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
-
Rivers, T. K. Filterable viruses: a critical review. J. Bacteriol. xiv, 217–258 (1927).
-
Carroll, D. et al. The global virome project. Science 359, 872–874 (2018).
-
Zhang, Y.-Z., Chen, Y.-M., Wang, Westward., Qin, X.-C. & Holmes, E. C. Expanding the RNA virosphere by unbiased metagenomics. Annu. Rev. Virol. vi, 119–139 (2019).
-
ICD-11 (World Wellness Arrangement, 2018).
-
Corman, Five. M., Muth, D., Niemeyer, D. & Drosten, C. Hosts and sources of endemic human coronaviruses. Adv. Virus Res. 100, 163–188 (2018).
-
González, J. M., Gomez-Puertas, P., Cavanagh, D., Gorbalenya, A. E. & Enjuanes, 50. A comparative sequence analysis to revise the current taxonomy of the family Coronaviridae. Curvation. Virol. 148, 2207–2235 (2003).
-
Saberi, A., Gulyaeva, A. A., Brubacher, J. L., Newmark, P. A. & Gorbalenya, A. East. A planarian nidovirus expands the limits of RNA genome size. PLoS Pathog. fourteen, e1007314 (2018).
-
Lai, Yard. M. C. Recombination in large RNA viruses: Coronaviruses. Semin. Virol. 7, 381–388 (1996).
-
Luk, H. K. H., Li, 10., Fung, J., Lau, South. K. P. & Woo, P. C. Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 71, 21–30 (2019).
-
Tao, Y. et al. Surveillance of bat coronaviruses in Kenya identifies relatives of human coronaviruses NL63 and 229E and their recombination history. J. Virol. 91, e01953–16 (2017).
-
Tao, Y. & Tong, S. 10. Complete genome sequence of a severe astute respiratory syndrome-related coronavirus from Kenyan bats. Microbiol. Resour. Ann. 8, e00548–19 (2019).
-
Hu, B. et al. Discovery of a rich factor puddle of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. xiii, e1006698 (2017).
-
Holmes, E. C. & Rambaut, A. Viral evolution and the emergence of SARS coronavirus. Philos. T. R. Soc. B 359, 1059–1065 (2004).
-
Hon, C. C. et al. Show of the recombinant origin of a bat astringent acute respiratory syndrome (SARS)-like coronavirus and its implications on the direct ancestor of SARS coronavirus. J. Virol. 82, 1819–1826 (2008).
Acknowledgements
Work on DEmARC advancement and coronavirus and nidovirus taxonomies was supported by the European union Horizon 2020 EVAg 653316 project and the LUMC MoBiLe program (to A.Due east.G.), and on coronavirus and nidovirus taxonomies by a Mercator Fellowship by the Deutsche Forschungsgemeinschaft (to A.E.G.) in the context of the SFB1021 (A01 to J.Z.).
We thank all researchers who released SARS-CoV-ii genome sequences through the GISAID initiative and particularly the authors of the GenBank MN908947 genome sequence: F. Wu, Due south. Zhao, B. Yu, Y. 1000. Chen, W. Wang, Z. G. Song, Y. Hu, Z. W. Tao, J. H. Tian, Y. Y. Pei, Thou. L. Yuan, Y. L. Zhang, F. H. Dai, Y. Liu, Q. G. Wang, J. J. Zheng, L. Xu, E. C. Holmes and Y. Z. Zhang. We thank S. G. Siddell, R. A. M. Fouchier, and J. H. Kuhn for their comments on a manuscript version posted on eleven February 2020 to bioRxiv. A.E.1000. and J.Z. thank W. J. Thousand. Spaan, A. J. Davison and E. J. Lefkowitz for support. A.E.One thousand. thanks members of the ICTV ExecutiveCommittee for discussions of classification and nomenclature issues relevant to this paper.
Author information
Affiliations
Consortia
Contributions
S.C.B., R.South.B., C.D., R.J.D.G., A.E.G., B.L.H., B.W.N., S.P., L.50.M.P., I.Southward. and J.Z. are members of the CSG, chaired by J.Z.; R. J.D.Thou., A.E.G., C.L., B.West.N. and J.Z. are members of the NSG, chaired by A.Due east.G.; A.E.G. and J.Z. are members of the ICTV. A.E.Chiliad., A.A.G., C.L., A.Thousand.L., D.P., D.5.S. and I.A.S. are members of the DEmARC team led past A.E.G. D.V.Southward. generated the nomenclature of SARS-CoV-2 using a computational pipeline developed by A.A.Thousand. and using software developed by the DEmARC team; the CSG considered and canonical this classification, and subsequently debated and decided on the virus proper noun. A.Due east.Thousand. and J.Z. wrote the manuscript. A.East.G. and D.V.S. generated the figures. All authors reviewed the manuscript and approved its submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's notation Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This commodity is licensed under a Artistic Commons Attribution 4.0 International License, which permits utilise, sharing, adaptation, distribution and reproduction in whatsoever medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The images or other third party material in this commodity are included in the commodity'southward Artistic Commons license, unless indicated otherwise in a credit line to the material. If textile is not included in the article'south Artistic Commons license and your intended employ is non permitted by statutory regulation or exceeds the permitted use, you lot will need to obtain permission straight from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Nigh this article
Cite this article
Coronaviridae Study Group of the International Commission on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 5, 536–544 (2020). https://doi.org/10.1038/s41564-020-0695-z
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/s41564-020-0695-z
Further reading
Source: https://www.nature.com/articles/s41564-020-0695-z
0 Response to "The Sars Virus Is a Member of the Family of Viruses"
ارسال یک نظر